BioCareSD
Our Customer Care Team and local, field-based account management teams are available 24 hours a day, 7 days a week, 365 days a year. We believe that trusted partnerships are pivotal to supporting patients’ needs. We are proud to support our partners in their mission to save lives.
We are always at your service.


Our Mission
We are committed to being the industry’s most trusted healthcare partner by providing customer-oriented solutions, unmatched service levels, and a unique distribution network to ensure the highest quality patient care.
Our Products
We feature an ever-expanding portfolio of specialty products for ophthalmology, hematology, thrombophilia, immunology, oncology, neurology, orphan and other ultra-rare diseases. BioCareSD is an Authorized Distributor of Record for all the manufacturers we represent, so you can depend on the quality of our products.
Wide-Reaching Distribution Capabilities
We have extremely agile and wide-reaching distribution capabilities to support our partners and their patients when they need it most.
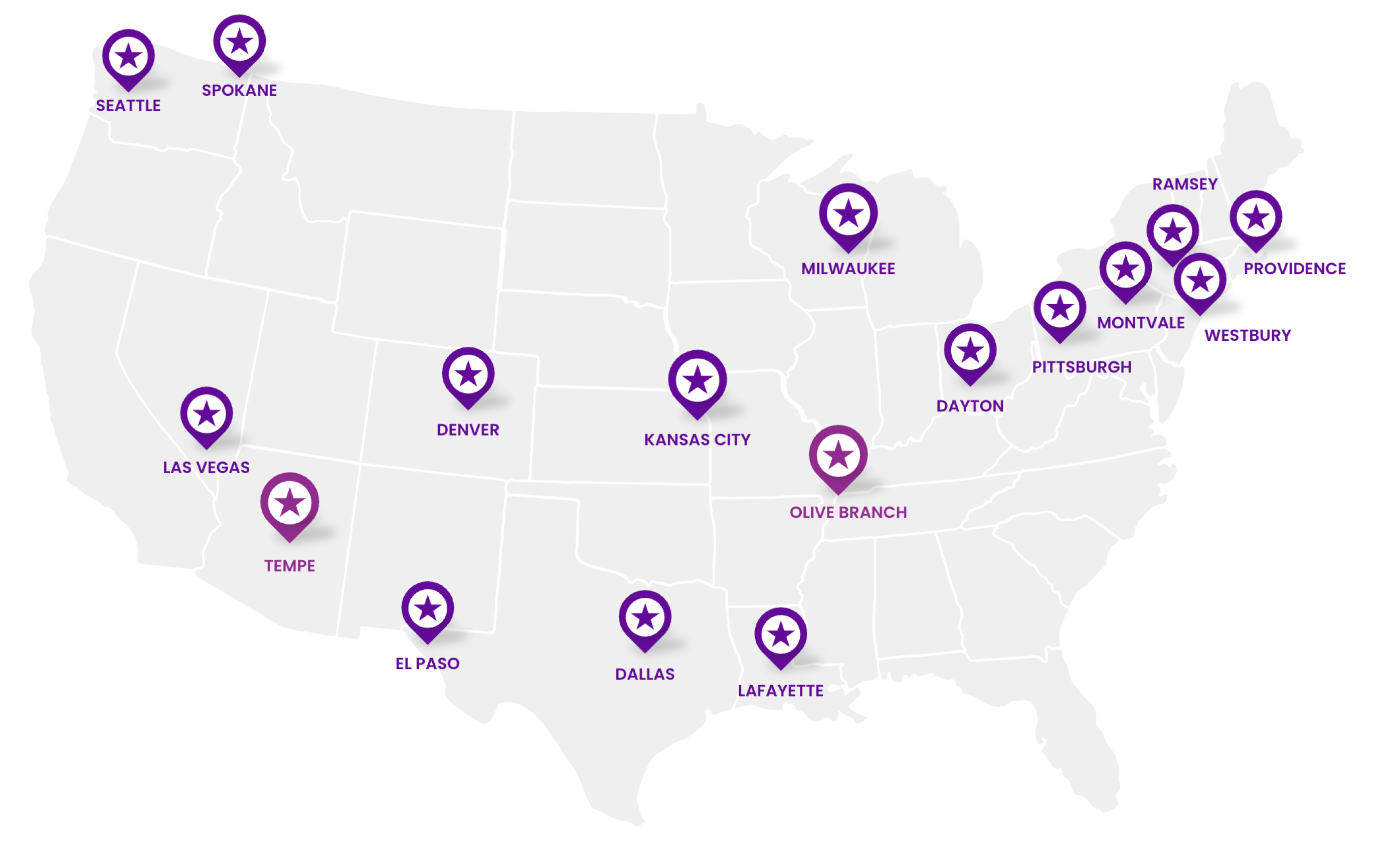
2 Strategically Located Distribution Centers (DCs)
15 Forward Stocking Locations
The BioCare Difference
The patient experience drives everything we do.
There is always a live BioCareSD team member to answer your calls 24/7/365.
Our distribution network is within a 6-hour delivery window.
Outstanding customer service IS who we are.




